The medical innovations in the past few years have been astounding. From life-saving medical devices to the FDA approved drugs that are revolutionizing healthcare, the new face of medicine has emerged. In fact, medical device revenues are reaching $5.5 billion by 2020. Noninvasive therapies, bioprinting, and reimagining drugs are just a few keeping the industry cutting-edge. Here are five healthcare game-changers to look for in 2020.
Let’s take a look.
Devices for Blood Collection and Diagnoses
Dr. Robert S. Langer, co-founder of Seventh Sense Biosystems, designed and developed TAP, an FDA-cleared device that collects blood using microneedles. Because the device makes the tiniest of punctures using microneedles, the process is virtually painless. According to Seventh Sense’s CEO, the needles puncture so rapidly that the patient doesn’t feel anything. The device is empowering; users just push a button to draw blood. Researchers are hoping that this will attract users, particularly the ones shy of blood. It’s a great resource for pharmacies and labs as well.
For basal and squamous skin cancer patients, TAP has been especially life-changing. It won the Dyson award for its dissolvable microneedles that treat cancer directly. Negar Tavassolian, director of the Stevens Bio-Electromagnetics Laboratory, has developed a technique to detect skin lesions and diagnose cancer. This has the ability to reduce painful and often unnecessary biopsies by 50 percent.
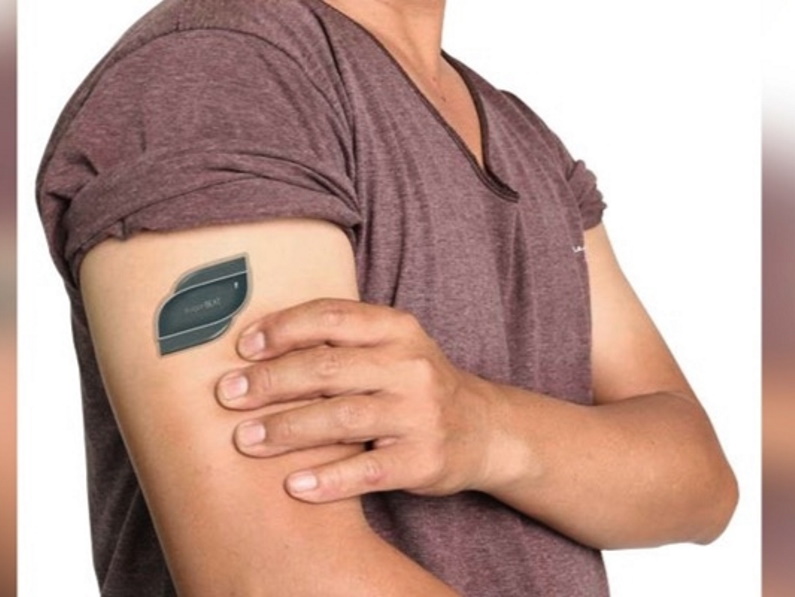
Perhaps one of the greatest medical innovations is the SugarBEAT®, a reusable electronic sensor that is applied using only a skin patch, began the first phase of commercial launch this year. This sensor continuously monitors glucose and get this – it’s BlueTooth-enabled. People with diabetes can now access their information anytime with the app. SugarBEAT® is being rolled out in the UK and Ireland in 2020.

A Medical Innovation for Peanut Allergies
Peanuts are one of the most common allergens today and are reported frequently in Australia, Western Europe, and in 0.6% of the United States population. While emergency epinephrine has greatly reduced severity, the development of a new oral immunotherapy drug may offer relief as well.
People with allergies have tried traditional oral immunotherapy before: eating gradually increasing doses of triggers. This is done in hopes of desensitizing individuals to the allergens. Unfortunately, it can still cause allergic reactions, even anaphylaxis.
Kari Nadeau, MD, PhD, professor of medicine and of pediatrics at Stanford and Sharon Chinthrajah, MD, professor of medicine and pediatrics, are the leads on this medical innovation. They’ve discovered that one injection of an antibody treatment called Etokimab, lets people with peanut allergies eat peanuts two weeks later. So far, the antibody is proving to be both safe and effective.
What’s great is that individually simply take the antibody treatment and eat it like normal a couple weeks later. These findings were published in November and researchers are hoping for a breakthrough in the coming year.
Scientists also announced another exciting oral immunotherapy this past September. It’s a capsule with a minuscule amount of peanut protein. It’s like traditional oral immunotherapy, but this study gave us a slightly better outcome: in a phase 3 clinical trial, 76.6% of children reached a daily maintenance dose of 300 mg (the equivalent of one peanut). Let’s see which therapy prevails in 2020.

Medical Innovations: Boundary-Pushing 3D and 4D Bioprinting
Just this year, Procter & Gamble signed a 3D bioprinting agreement with the biotechnology company Aether. Collaborators are hoping to advance the use of artificial intelligence (AI) in 3D bioprinting.
Aether is the developer of the Aether 1 3D bioprinter, which has changed the face of synthetic biology. The bioprinter allows doctors to create cellular scaffolding for biomedical uses. It’s a machine that can use almost any tool, and its 8 syringe extruders can develop complex tissues called bioinks. So far, scientists have applied their research in organ replacement, stem cells, orthology, and even wound treatment.
But the CEO of Aether believes that AI will redefine the entire bioprinting realm. The new medical innovation will use AI algorithms so that monitoring and printing are similar to commercial manufacturing processes. Researchers will use AI to automate image processing software to find faulty prints.
Another incredible boundary-pushing bioprinting is 4D printing. Going an extra step in the process, printed biomedical materials can actually have their shape changed or behavior altered in response to certain stimuli. Think of a printed heart or even an intestine that contracts. Thankfully, laboratories have even been able to reduce their need for animal testing.

First-ever Treatment for a Fatal Cardiomyopathy
Transthyretin amyloid cardiomyopathy is a rare and progressively debilitating disease that not many know about. It’s where proteins called amyloid fibrils deposit and accumulate in the walls of the heart’s left ventricle. Misfolded transthyretin protein makes up these deposits, and they stiffen the muscle, which then causes heart failure.
When it affects the heart, patients suffer from shortness of breath, fatigue, heart failure, loss of consciousness, abnormal heart rhythms and death.
In May of this year, the FDA approved Pfizer’s newly developed tafamidis, for this specific condition. The drug binds to transthyretin and prevents the misfolding of the deposited protein. Exciting clinical trial results showed that patients on tafamidis had a 30% lower mortality rate as well as decreased cardiovascular-related hospitalizations.
2019 was special for transthyretin amyloid cardiomyopathy because it marked the FDA approval of tafamidis, the first-ever medication for treatment.

A New Therapy for Ovarian Cancer
One of the most recent advances in ovarian cancer treatment was the FDA approval of poly-ADP ribose polymerase (PARP) inhibitors, a novel class of first-line maintenance therapy drugs in advanced-stage ovarian cancer.
PARP inhibitors are a class of enzymes that are activated by DNA damage. Researchers originally designed them for women with ovarian cancer associated with mutations involving the BRCA genes (mBRCA).
Three clinical trials have made incredible strides in improving outcomes. At the European Society for Medical Oncology (ESMO) Congress 2019, researchers presented their findings. The studies tested the PARP inhibitors niraparib, olaparib, and veliparib, on women with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Traditionally for most patients, cancer returns, but these therapies substantially delayed the length of time before it came back.
It’s significant because it’s the first time achieving prolonged progression-free survival, and it’s particularly beneficial for women with homologous recombination deficient (HRD) tumors.
Overall, the results presented showed a 70% reduction in the risk of disease progression or death at 3 years, when using a PARP inhibitor. The addition of PARPs as a medical innovation in 2020 for treating ovarian cancer is undoubtedly beneficial to women and the medical industry greatly anticipates next year’s research.

The Emergence of Medical Innovations in 2020
The face of healthcare is constantly changing with the advances in technological innovations. In 2020, artificial intelligence will certainly be a force to be reckoned with in the medical industry. It’s exciting to see the new medical innovations and approaches researchers are taking when developing and designing drugs and you can’t help but wonder what’s coming as we wrap up yet another decade.